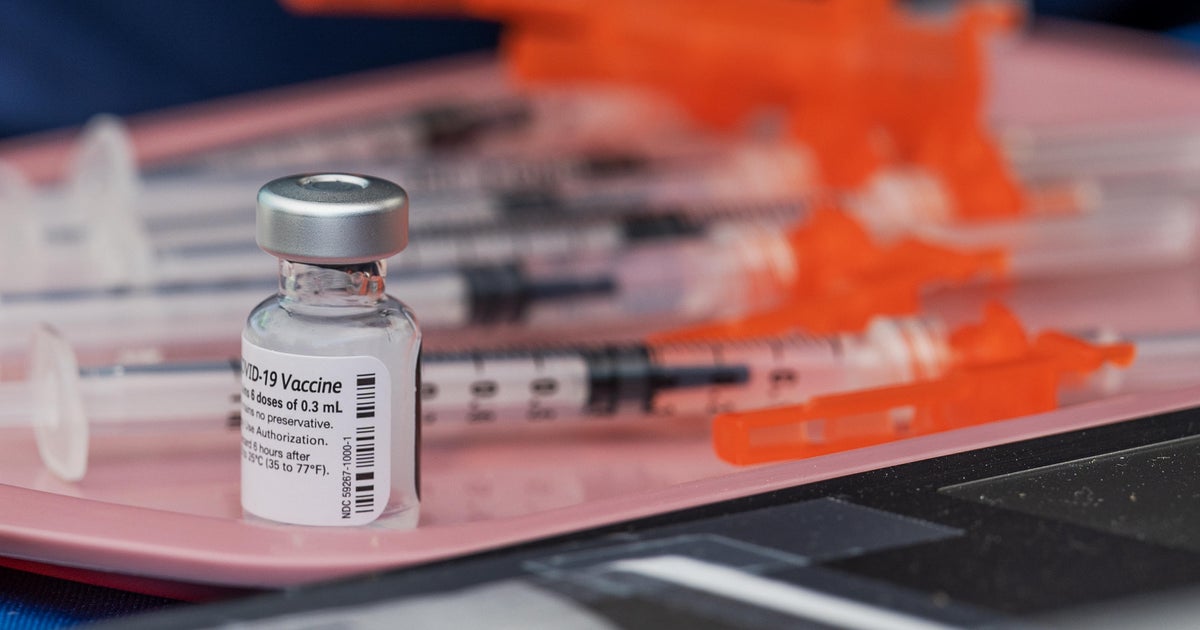
A top-ranking Food and Drug Administration official, responsible for overseeing the approvals of the new vaccines now rolling out for this fall and winter’s three respiratory virus threats, said this month he is personally planning to space out his vaccinations over the coming weeks.
“Some people are saying, ‘Well, could I get RSV, COVID and the flu vaccine on the same day?’ Yes, indeed, you could. But honestly, I might not,” said Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research.
Instead, he said he intended to get the COVID shot right away and the flu shot in early October.
Marks, who was speaking during a recent call with FDA stakeholders, stressed that he did not disagree with guidance from the Centers for Disease Control and Prevention which allows giving multiple different routine shots during the same visit. Doctors refer to this as “coadministration” or “simultaneous administration” of vaccines.
However, he acknowledged that getting up to three of the different vaccines at the same time could lead to more side effects — like stronger fatigue or a small fever — in the days after getting the shots.
Spacing out the shots by around two weeks could “minimize the chance of interactions, and minimize confusing side effects from one with another,” he said. They might be a good option for people who did not mind multiple trips to the pharmacy or their doctor’s office.
“I might just want to space them out a little bit. But if you had to drive a lot of miles to get the vaccines, then it might not be unreasonable to get all three of them at once,” said Marks.
Getting an updated COVID-19 vaccine now
Of the currently available options, Marks said that his plan was to get a dose of the updated COVID-19 vaccine first.
Health authorities have been fortunate to have a vaccine that appears likely to work well for protecting against the currently circulating strains of the virus, he said.
“It’s like having a bird in the hand. I have a bird in the hand, good match, a lot of COVID around, great time to go get vaccinated,” said Marks.
The FDA had selected the strain to target in the current batch of shots back in June, clearing the way for vaccine makers to ramp up their production ahead of a fall rollout.
Marks cited recent data suggesting that these updated vaccines, which have been revised from previous designs to now target the XBB.1.5 strain of the virus, would also work to boost protection against its closely-related descendants now dominant nationwide.
Early results shared by the vaccine makers with a CDC panel earlier this month also suggests that these updated shots will also work against the highly mutated BA.2.86 variant, which has been reported in a growing number of states.
Several leading COVID trends, like emergency department visits, have started to slow in recent weeks following a summer wave that began to accelerate last month. Another “moderate” wave is predicted to begin over the coming colder months, the CDC’s disease forecasters say, with that surge’s peak expected to arrive potentially earlier than it did last season.
Marks said it was possible that health authorities might allow for another dose to be offered to some vulnerable groups later in the fall and winter.
“I think if we saw that it appeared that people might benefit in a few months from an additional dose, we would probably work with our CDC colleagues to issue a recommendation at that time. But right now we are just talking about a single recommendation, a single dose,” he said.
Scheduling a flu shot for early October
By early next month, Marks said he plans to have received his flu shot.
“I usually get my influenza vaccine around October 1st,” Marks said.
This is later than some other health officials within the Biden administration. CDC Director Dr. Mandy Cohen posted on social media on Sept. 6 to say she had gotten vaccinated for the flu.
Similar to previous seasons, CDC’s official recommendations for this year are that “[flu] vaccination should ideally be offered during September or October.”
Marks said that the boost in protection offered by flu vaccines can wane, underscoring why the shots should not be given too early in the season, before the threat of infections ramps up. Flu season in the U.S. typically peaks between December and February, but can stretch into the spring.
The protection from a flu shot “has a little bit of a shorter life than we might like, in some ways it’s a little like the COVID vaccines,” Marks said.
Right now, weekly CDC data suggests flu activity remains at low levels in most parts of the country.
New options for RSV
Some Americans also have new options to be immunized for RSV, or respiratory syncytial virus, for the first time this year.
Older adults, ages 60 and older, can get a dose of the new vaccines developed by Pfizer or GSK. The CDC recommends that shots be offered “as early as vaccine supply becomes available” this year.
CDC data suggests RSV infections have begun to accelerate in some parts of the country, with the steepest rises in the Southeast.
A panel of CDC advisers is scheduled to vote Friday on updated recommendations on giving Pfizer’s new RSV vaccine during pregnancy as well, in hopes of passing on protection to newborns during their most vulnerable early months of life.
A new antibody injection from Sanofi and AstraZeneca is also available for babies this year, which is recommended to be given to infants born ahead of this coming RSV season.